General Approach
- Optimise underlying condition[1]
- Breathing SPACE[2]
- Smoking cessation Ask about smoking, advise to quit, offer assistance to quit. Adopt the CO4 approach:
- The right COnversation, with every patient and staff member who smokes, that gives them a chance to quit, referring for support if necessary
- Offer routine exhaled carbon monoxide (CO) monitoring: “Would you like to know your level?”
- COde smoking cessation interventions and include smoking history in death certification
- COmmission services where smoking cessation behaviours are incentivised systematically
- Pulmonary disease
- Ask about symptoms—cough, sputum, variability, nocturnal symptoms, chest discomfort, haemoptysis
- Investigations—rapid access to quality assured spirometry
- Pulse oximetry
- Prioritise high-value interventions—smoking cessation, pulmonary rehabilitation, flu vaccination
- Ensure that inhaled medications are both prescribed and used appropriately
- Anxiety
- Psychosocial factors contribute to symptoms in cardiorespiratory disease. Anxiety may present with specific features of hyperventilation/dysfunctional breathing syndrome, including paraesthesia and “air hunger”
- Parity of esteem—address the physical health of people with mental health issues
- Smoking cessation interventions are effective and safe to use in people with mental health problems
- Peer support, e.g., BLF Breathe Easy groups
- Cardiac disease
- Ask about risk factors (smoking, hypertension, diabetes, ischaemic heart disease?)
- Abnormal pulse, pulmonary crepitations, oedema, cardiac murmurs
- Cardiac complications of respiratory disease—pulmonary hypertension, sleep-disordered breathing
- Investigations—consider ECG, BNP, echocardiogram
- Refer patients with heart disease who feel limited by their symptoms for exercise rehabilitation
- Exercise level and fitness
- Ask about exercise level “Do you take any regular exercise?”
- Give brief advice to increase physical activity levels
- Reassure and encourage: “It’s not harmful to make yourself breathless”
- Refer patients with lung or heart disease who feel limited by their symptoms for exercise rehabilitation
- Obesity—identify this explicitly as a contributor to breathlessness
- Signpost opportunities to participate in exercise (e.g., Park Run, Couch to 5K). Pedometer-based interventions with a step count goal are effective
- Prioritise evidence based non-pharmacological interventions prior to pharmacological interventions. [3]
- Smoking cessation Ask about smoking, advise to quit, offer assistance to quit. Adopt the CO4 approach:
Disease oriented reversibility
Malignancy
-
- The evidence base for managing breathlessness in patients with advanced cancer is smaller compared with the evidence base for patients with chronic obstructive pulmonary disease and congestive heart failure. Although some of this evidence may be applicable to advanced cancer, breathlessness in patients with advanced cancer has unique characteristics. In a study of 70 patients with advanced cancer, breathlessness was constant in 39% (with additional breakthrough episodes) and episodic (largely <10 minutes) in 61% of patients.
| Condition | Management Strategies |
| Anaemia (symptomatic) | Consider transfusion if haemoglobin <70–80 g/L to keep haemoglobin above 70–80 g/L |
| Asthma/COPD exacerbation | Medical optimisation |
| Cachexia | Consider referral to palliative care, dietician and/or physical therapy |
| Central airway obstruction | For proximal lesions, consider endobronchial interventions, such as bronchoscopy with mechanical
debridement, tumour ablation and airway stent placement For distal lesions, consider radiotherapy |
| Cytotoxic chemotherapy-induced pulmonary toxicities | Withhold treatment and consider corticosteroids |
| Immunotherapy-induced
pulmonary toxicities |
Withhold treatment and consider corticosteroids |
| Heart failure exacerbation | Medical optimisation |
| Lymphangitic carcinomatosis | Treatment of underlying malignancy. Consider corticosteroids (anecdotal) |
| Malignant ascites | Paracentesis with or without indwelling catheter |
| Malignant pleural effusions | For patients with a short-life expectancy (<3 months), consider simple thoracentesis
For patients with longer life expectancy, consider tunnelled pleural catheter or chemical pleurodesis; both are reasonable options |
| Malignant pericardial effusion/
tamponade |
Pericardiocentesis, pericardiectomy with or without pericardial window |
| Metabolic acidosis | Identify and treat the underlying cause |
| Pneumonia | Anti-infective agents |
| Pulmonary embolism | Anticoagulation |
| Radiation-induced pneumonitis
or fibrosis |
Treatment of underlying malignancy. Consider corticosteroids (anecdotal) |
| Tumour embolism | Treatment of underlying malignancy |
Heart Failure
- Offer a mineralocorticoid receptor antagonists (MRA), in addition to an ACE inhibitor (or ARB) and beta-blocker, to people who have heart failure with reduced ejection fraction if they continue to have symptoms of heart failure.[4]
- Consider sacubitril/valsartan as an option for treating symptomatic heart failure with reduced ejection fraction in people:
- with New York Heart Association (NYHA) class II to IV symptoms and
- with a left ventricular ejection fraction of 35% or less and
- who are already taking a stable dose of angiotensin converting enzyme (ACE) inhibitors or ARBs.
- Diuretics should be routinely used for the relief of congestive symptoms and fluid retention in people with heart failure, and titrated (up and down) according to need following the initiation of subsequent heart failure therapies.
- People who have heart failure with preserved ejection fractionshould usually be offered a low to medium dose of loop diuretics (for example, less than 80 mg furosemide per day). People whose heart failure does not respond to this treatment will need further specialist advice. [4]
- Implantable cardioverter defibrillators (ICDs), cardiac resynchronisation therapy (CRT) with defibrillator (CRT‑D) or CRT with pacing (CRT‑P) are recommended as treatment options for people with heart failure who have left ventricular dysfunction with a left ventricular ejection fraction (LVEF) of 35% or less as specified below[5]:
| NYHA class | ||||
| QRS interval | I | II | III | IV |
| <120 milliseconds | ICD if there is a high risk of sudden cardiac death | ICD and CRT not clinically indicated | ||
| 120–149 milliseconds without LBBB | ICD | ICD | ICD | CRT‑P |
| 120–149 milliseconds with LBBB | ICD | CRT‑D | CRT‑P or CRT‑D | CRT‑P |
| ≥150 milliseconds with or without LBBB | CRT‑D | CRT-D | CRT‑P or CRT‑D | CRT‑P |
| LBBB, left bundle branch block; NYHA, New York Heart Association | ||||
Respiratory Disease
Respiratory symptoms are ubiquitous and impair health-related quality of life in people with respiratory disease. The European Respiratory Society (ERS) recently published evidence-based recommendations for symptomatic treatment in people with serious respiratory illness:
Clinical Practice Guidelines Recommendations
| Clinical Practice Guideline Question | Recommendation |
| Question 1: Should a multicomponent service be used to reduce symptoms in people with serious respiratory illness? | We suggest that multicomponent services should be used to reduce symptoms in people with serious respiratory illness (conditional recommendation, very low certainty of evidence). |
| Question 2: Should graded exercise therapy be used to reduce fatigue in people with serious respiratory illness? | We suggest that graded exercise therapy be used to reduce fatigue (conditional recommendation, low certainty of evidence). |
| Question 3: Should increased airflow be used to reduce breathlessness in people with serious respiratory illness? | We suggest the use of increased airflow to reduce breathlessness in people with serious respiratory illness (conditional recommendation, very low certainty of evidence). |
| Question 4: Should supplemental oxygen be used to reduce symptoms in people with serious respiratory illness? | We suggest either administering or not administering supplemental oxygen to reduce symptoms in people with serious respiratory illness (conditional recommendation, low certainty of evidence). |
| Question 5: Should opioids be used to reduce symptoms in people with serious respiratory illness? | We suggest not using opioids for the treatment of breathlessness in people with serious respiratory illness (conditional recommendation against the intervention, very low certainty of evidence). |
| Question 6: Should breathing techniques be used to reduce symptoms in people with serious respiratory illness? | We suggest that breathing techniques be used to reduce symptoms in people with serious respiratory illness (conditional recommendation, very low certainty of evidence). |
| Question 7: What is the role of needs assessment tools in people with serious respiratory illness? | We suggest that needs assessment tools may be used as part of a comprehensive needs assessment, but do not replace patient centred care and shared decision making (conditional recommendation, low certainty of evidence). |
Reproduced with permission of the ERS 2024 [6]
Pleural Effusions
- PLEASE trial evaluated breathlessness in patients with symptomatic pleural effusion pre and post therapeutic drainage
- Drainage provided meaningful breathlessness relief (VAS score improved ⩾14 mm) in 73% of participants irrespective of whether the lung expanded or not.
- Factors associated with breathlessness relief were: significant breathlessness pre-drainage (odds ratio (OR) 5.83 per standard deviation (SD) decrease); baseline abnormal/paralyzed/paradoxical diaphragm movement (OR 4.37); benign aetiology (OR 3.39); higher pleural pH (OR per SD increase 1.92); and higher serum albumin level (OR per SD increase 1.73).
- Breathlessness and exercise tolerance improved in most patients with only a small mean improvement in spirometry and no change in oxygenation. Breathlessness improvement was similar in participants with and without trapped lung. Abnormal hemi-diaphragm shape and movement were independently associated with relief of breathlessness post-drainage.[7]
COPD Specific
- Smoking cessation is key – up to 40% of patients with COPD continue to smoke Non-pharmacological – as per Breathing Space
- Inhaled medications
- Beta 2 agonists
- Short acting beta2 agonists (SABA) and Long acting beta2 agonists (LABA) both significantly improve FEV1 and symptoms of dyspnoea
- LABAs significantly improve FEV1 and lung volumes, dyspnea, health status, exacerbation rate and number of hospitalizations, but have no effect on mortality or rate of decline of lung function
- Stimulation of beta2-adrenergic receptors can produce resting sinus tachycardia and has the potential to precipitate cardiac rhythm disturbances in susceptible patients. Exaggerated somatic tremor is troublesome in some older patients treated with higher doses of beta2-agonists, regardless of route of administration
- Antimuscarinic drugs
- Antimuscarinic drugs block the bronchoconstrictor effects of acetylcholine on M3 muscarinic receptors expressed in airway smooth muscle.
- A systematic review of randomized controlled trials concluded that ipratropium, a short acting muscarinic antagonist, alone provided small benefits over short-acting beta2-agonist in terms of lung function, health status and requirement for oral steroids.
- LAMA treatments improve symptoms, including cough and sputum and health status. They also improve the effectiveness of pulmonary rehabilitation and reduce exacerbations and related hospitalizations. Clinical trials have shown a greater effect on exacerbation rates for LAMA treatment (tiotropium) versus LABA treatment.
- Main side effects include dry mouth
- Combination therapies
- Combination of SABA and SAMA are superior to either as monotherapy
- Combination of LABA and LAMA increases FEV1 and reduces exacerbations compared to monotherapy
- Triple inhaled therapy of LABA+LAMA+ICS improves lung function, symptoms, and health status whilst reducing exacerbations compared to other combined inhalers.
- There is high quality evidence from randomized controlled trials that ICS use modifies the airway microbiome and is associated with higher prevalence of oral candidiasis, hoarse voice, skin bruising and pneumonia.
- Oxygen
- Long-term oxygen therapy (LTOT) is indicated for stable patients who have:
- PaO2 at or below 55 mmHg (7.3 kPa) or SaO2 at or below 88%, with or without hypercapnia confirmed twice over a three-week period
- PaO2 between 55 mmHg (7.3 kPa) and 60 mmHg (8.0 kPa), or SaO2 of 88%, if there is evidence of pulmonary hypertension, peripheral edema suggesting congestive cardiac failure, or polycythemia (hematocrit > 55%).
- Once placed on LTOT the patient should be re-evaluated after 60 to 90 days with repeat arterial blood gas (ABG) or oxygen saturation measurements while inspiring room air and the level of oxygen flow that had been prescribed to determine if oxygen is still indicated and if so, therapeutic.[8]
- Long-term oxygen therapy (LTOT) is indicated for stable patients who have:
- Beta 2 agonists
Non-pharmacological
Breathing, Thinking, Functioning (BTF) model
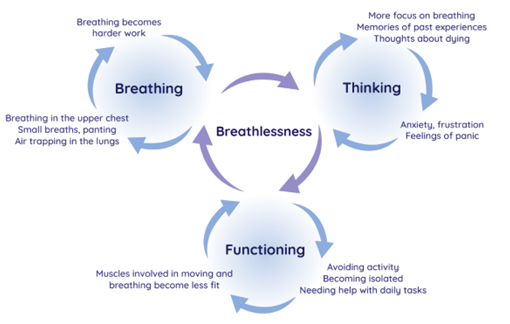
- Breathing domain
- Dysfunctional breathing patterns are well recognised in breathless individuals without underlying respiratory pathology. Features include apical breathing, higher ratio of inspiratory to expiratory length, absence of end-expiratory pause and frequent sighs or yawns. Breathing pattern disorders occur in about a third of asthma patients, and although there has been no research in other respiratory conditions, such as COPD, there is extensive anecdotal evidence that dysfunctional breathing patterns do occur. Breathless patients experience a sense of ‘needing more air’ and, therefore, may consciously or subconsciously increase their tidal volume or respiratory rate, breathing predominantly using the upper chest and accessory muscles. Apical breathing causes reliance on fatiguable accessory muscles of respiration, underutilising the efficient and relatively fatigue-resistant diaphragmatic muscle, further increasing the work of breathing and intensifying breathlessness.
- Interventions specific to problems with the breathing domain include:
- Breathing Techniques
- Handheld fan use
- Airway Clearance Techniques
- Inspiratory muscle training
- Chest wall vibration
- Non-invasive ventilation
- Thinking domain
- It is well-recognised that the anxiety or fear caused by breathlessness can, in turn, augment the perception of breathlessness. This vicious cycle can lead to a rapidly escalating sense of panic, which most breathless patients experience at some point. The neural processing for this feedback loop is likely to occur in the cortico-limbic areas of the brain, involved in both breathlessness perception and processing of emotion. In addition, anxiety increases the respiratory rate and can cause muscle tension in both the ventilatory pump and other skeletal muscles, so further increasing the work of breathing and respiratory demand.
- Interventions specific to problems with the thinking domain include:
- Cognitive behavioural therapy
- Relaxation techniques
- Mindfulness
- Acupuncture
- Functioning domain
- As breathlessness is so unpleasant, it is natural to avoid it by reducing activity. However, inactivity leads to muscle deconditioning, with reduced oxidative capacity, muscle fibre atrophy and fibre type shift from type 1 (oxidative) to type IIb (glycolytic) fibres. This increases the demand on the respiratory system and worsens breathlessness further. Patients intuitively understand this ‘deconditioning vicious cycle’, being aware that less ‘fit’ people are more breathless on exertion. Family members and other carers can unwittingly compound the situation, by trying to help through doing activities that the patient might otherwise have done.
- Interventions specific to problems with the functioning domain include:
- Pulmonary rehabilitation
- Activity promotion
- Walking aids
- Pacing
- Neuromuscular electrical stimulation
- Strategies to tackle patient misconceptions at each stage.

Education/Self management
- For patients receiving curative treatment, education is often framed around enhancing recovery and returning to a healthy lifestyle. In advanced cancer, where deterioration is expected, it can be framed around making the most of the present and staying independent for as long as possible. Patients should be assured that being breathless is itself not dangerous, and that breathlessness is a normal exertional response that settles with rest. Even following incremental exercise to a symptom limited maximum, breathlessness in people with lung cancer typically recovers within a few minutes.
- Advice on positions to aid recovery following exertion, or during an episode of breathlessness, can be useful. A ‘forward lean’ position may reduce accessory muscle work, improving diaphragm function and ventilatory capacity. Relaxed sitting (with hands or elbows rested on thighs) or standing (using a wall as support) and high side lying (supporting head and chest) are frequently recommended within breathlessness services.[3]
Air Flow Therapy
- Cooling of the facial skin innervated by the second and third branches of the trigeminal nerve, nasal mucosae or the upper airway flow receptors could modulate the central perception of breathlessness leading to decreased neural respiratory drive, thereby reducing the sensation of breathlessness.[9]
- Systematic reviews of oxygen in a variety of non-hypoxic patient groups (cancer, chronic heart failure, kyphoscoliosis, chronic obstructive pulmonary disease and interstitial lung disease) have not demonstrated additional benefit from oxygen therapy over medical air delivery.[9]
- In a recent meta-analysis and systematic review they found that current data supports that facial and nasal airflow delivery at rest offers relief of breathlessness intensity consistent with a moderate clinically important difference and during exertion. All participants in the cylinder medical air delivery at rest studies had advanced cancer, but nearly half of those in the fan ‘at rest’ studies had other conditions, indicating that airflow for breathlessness at rest is of benefit irrespective of cause.[9]
- In a meta-analysis focusing specifically on patients with advanced cancer, fan therapy was associated with short-term (5 minutes) improvements in breathlessness – however this was not reflected in longer term breathlessness control ( > 1 month).[10]
- A recent small scale study of different fan designs in relieving exercise induced breathlessness suggested that a slower rate of airflow (2.85m/s) was better tolerated than a faster rate of airflow (4.91m/s). [11]
Non-invasive ventilation
- In an intensive care setting, an RCT comparing bilevel ventilation versus standard oxygen therapy found a statistically significant improvement in breathlessness in the bilevel ventilation group vs standard supplemental oxygen group[10]
- NIV also appears to improve breathlessness in the palliative setting; however, potential harms include facial pressure injuries, claustrophobia and anxiety.[12]
- The use of NIV demonstrated effectiveness in managing daily breathing problems when incorporated into the daily routines of ALS patients. As the disease progresses, there is an observed increase in the prevalence of NIV use among ALS patients. In the final stages of the disease, specifically during the last 24 h of life, one third of ALS patients were reported to have utilized NIV.[13]
Oxygen
- In patients with advanced cancer, supplemental oxygen is not recommended when SpO2 >90%[14]
- Oxygen has not been consistently shown to relieve breathlessness in the palliative setting in patients with mild or no hypoxaemia in advanced disease[3]
- In patients with chronic breathlessness despite other evidence-based treatments, a trial of supplemental oxygen could be justified, based on indirect data that supplemental oxygen can improve exercise capacity and breathlessness observed during exercise testing in the laboratory for patients with COPD and interstitial lung disease. Individualised information and shared decision-making with patients and caregivers are important. If tried, supplemental oxygen should be reevaluated within a few days and discontinued if the patient perceives no benefit.[3]
- Palliative oxygen continues to be initiated for the management of breathlessness in non-hypoxaemic patients with advanced life-limiting illnesses despite growing evidence for its inappropriateness. [15]
- In patients with COPD, a 2016 Cochrane review was moderately confident that oxygen can relieve breathlessness when given during exercise to mildly hypoxaemic and non-hypoxaemic people with chronic obstructive pulmonary disease who would not otherwise qualify for home oxygen therapy. Most evidence pertains to acute effects during exercise tests, and no evidence indicates that oxygen decreases breathlessness in the daily life setting. Findings show that oxygen does not affect health-related quality of life.[16]
- Similarly, in a four-arm, double-blind, randomized clinical trial examining the role of high-flow nasal cannula on exertional dyspnoea in patients with cancer without hypoxemia, high-flow oxygen, but not high-flow air, resulted in significantly lower dyspnoea scores and longer exercise time. High-flow oxygen delivered by high-flow nasal cannula devices may improve clinically relevant outcomes even in patients without hypoxemia.[17]
Mobility Aids
- Mobility aids help to improve both breathlessness and mobility through an increased ventilatory capacity and/or reduced metabolic cost.
- Several randomised crossover studies in breathless patients with COPD demonstrate that use of a rollator improves self-paced walking distance in both indoor and outdoor environments, especially in patients severely limited by breathlessness, including those using ambulatory oxygen.[3]
Exercise
- Pulmonary rehabilitation has been well-established as a treatment for breathlessness in COPD, producing improvement in patient-reported dyspnoea as well as in exercise capacity. [18]
- In a large, three-arm randomized controlled trial in patients with late-stage lung cancer compared two widely practiced physical activity modalities, Tai Chi and aerobic exercise in terms of their impact on cancer-related dyspnoea outcomes. Findings indicated that, compared to the control group, participants in the Tai Chi group demonstrated a significant improvement in overall dyspnoea levels immediately following the intervention, and this positive effect was sustained even after eight months. Both Tai Chi and aerobic exercise demonstrated significant reductions in lung cancer-specific dyspnoea compared with the control group at eight-month post intervention.[19]
- Unsupervised physical activity interventions benefits dyspnoea and exercise capacity of people with COPD, are safe and present a high adherence rate. [20]
Multicomponent interventions
- In patients with advanced cancer, the combination of behavioural/psychoeducational, activity/rehabilitation, and integrative medicine interventions had better association with improved breathlessness compared with usual care.[10]
- In a systematic review of holistic, symptom-focused complex interventions in patient with advanced disease, patients and carers valued tailored education, self-management interventions and expert staff providing person-centred, dignified care. However, there was no observable effect on health status or quality of life, and mixed evidence around physical function. Holistic services for chronic breathlessness can reduce distress in patients with advanced disease and may improve psychological outcomes of anxiety and depression.[21]
Acupuncture/acupressure
- Acupunctureis a widely used technique in traditional Chinese medicine (TCM) and numerous randomized controlled trials have evaluated the clinical efficacy of acupuncture. The concept of TCM is based on the harmonious flow of “Qi,” commonly translated as “life energy.” Disease or distress occurs if the continuous flow of Qi in some of these meridians is somehow disrupted. Specific acupuncture points (acupoints) are located on the meridians. Stimulation of these points by needles (acupuncture), finger pressure (acupressure), or other methods (laser, electric stimulation) may restore the continuous flow of energy and may maintain health by enhancing robustness.[22]
- In a recent systematic review and meta-analysis there was a significant effect for acupuncture reducing breathlessness severity in patients with advanced diseases. Furthermore, the meta-analysis on the 6MWT (6 minute walk test) showed a significant treatment effect of acupuncture compared to the control group. [22]
- In patients with advanced cancer, a therapeutic trial of acupressure or acupuncture can be considered according to patient preference[3][14]
Pharmacological
Opioids
Summary
- An excellent review of the current evidence base of the benefits and disadvantages of the use of opioids in breathlessness management can be found here.[23]
- Due to limitations in the study design for MORDYC and BEAMS (see below), the MABEL study is currently underway to evaluate whether long-acting morphine improves breathlessness symptoms in those with moderate to severe chronic breathlessness in the longer term compared to placebo. [24]
COPD
- In 2020, the Morphine for Treatment of Dyspnea in Patients With COPD (MORDYC) study, a randomized, double-blind, placebo-controlled, parallel-arm intervention study of 111 patients with COPD and moderate to very severe breathlessness, showed that regular, low-dose, oral extended-release (ER) morphine for 4 weeks was clinically relevant and statistically significant for improving disease-specific health status[25]
- However the BEAMS (Breathlessness, Exertion, And Morphine Sulfate) trial including 156 participants, did not show an improvement in their primary outcome, worst breathlessness after 1 week of treatment, or in other outcomes. The study findings may not be applicable to people with very advanced COPD and breathlessness who are in palliative care or near the end of life, at which time treatment with opioids may be useful to provide relief of severe dyspnoea. [26]
- Subsequent systematic reviews suggest that short acting opioids appeared to be more safe, have potential to lessen dyspnoea and improve exercise endurance, supported benefit in managing episodes of breathlessness and providing prophylactic treatment for exertional dyspnoea.[27]
Heart failure
- A recent systematic review concluded that opioids for treating breathlessness in HF are questionable and may only be the very last option if other options have failed or in case of an emergency. [28]
Pulmonary Arterial Hypertension
- In a small, randomised, cross-over study of extended-release (ER) morphine versus placebo, results favoured placebo over ER morphine in every important outcome measure and that ER morphine generated consistently more harms.[29]
Motor Neurone Disease/Amyotrophic Lateral Sclerosis
- Opioids have been employed to manage breathlessness in ALS patients towards the end of life, with notable patterns observed a significant majority of non-cancer ALS patients received as-needed opioids in the last 24 hours before death. However, only three out of four ALS patients had regular prescriptions for opioids to address breathlessness; less than half of the ALS patient population received opioid treatment specifically for dyspnoea. [13]
Cancer
- A recent systematic review concluded that the effect of opioids on the relief of cancer-related dyspnoea was significant with a standardized mean difference of − 0.43, with no significant increase in serious adverse events with opioids compared to placebo or other drugs. [30]
- There are few RCTs to inform use of opioids for treatment of breathlessness in patients with cancer already taking regular opioids for pain. Several small placebo-controlled trials have examined the use of prophylactic fentanyl given prior to exertion in opioid-tolerant patients with cancer and reported a benefit; taken together, the available evidence signals that opioids have a positive effect on breathlessness among opioid-tolerant individuals. [3]
- Recent large-scale observational study found that, in patients with cancer starting opioids for breathlessness, intensity of both subjectively and objectively measured dyspnoea improved over a period of 72 hours, with 82% responding after 72 hours and a mean improvement on NRS of -2.47. The most common adverse events were somnolence (29%), nausea (5%) and delirium (15%). The factors related to the response of dyspnoea improvement by systemic opioids were opioid-naivety, the absence of liver metastasis, CPS of weeks or months, and severe levels of dyspnoea (NRS ≥6).[31]
- Parenteral morphine, oxycodone, and hydromorphone may be similarly effective and safe for cancer patients with terminal dyspnoea. [32]
- Very low‐quality evidence means that no conclusions can be drawn regarding the effects of opioids compared with placebo in adults with advanced disease and terminal illness. Patients with advanced disease and terminal illness (including severe chronic obstructive pulmonary disease, heart failure, or advanced cancer) who received opioids primarily morphine) for refractory breathlessness showed no consistent improvement in their symptoms compared with those receiving placebo; researchers observed an improvement in endpoint scores, but not when baseline scores were taken into account. Analyses of exercise tolerance (measured on a range of scales, including the six‐minute walk test) and quality of life (measured by the Chronic Respiratory Disease Questionnaire) were highly underpowered and inconsistent. Very low‐quality evidence suggests that rates of constipation, nausea and vomiting, and drowsiness may be higher with opioids than with placebo.[33]
Benzodiazepines
- There is no evidence for or against benzodiazepines for the relief of breathlessness in people with advanced cancer and COPD. Benzodiazepines caused more drowsiness as an adverse effect compared to placebo, but less compared to morphine. Benzodiazepines may be considered as a second- or third-line treatment, when opioids and non-pharmacological measures have failed to control breathlessness.[34]
- In the last days of life, some patients may continue to experience severe breathlessness despite maximising all other palliative measures within the limited time frame. In these unique situations, palliative sedation may be considered to alleviate suffering. Benzodiazepines such as midazolam infusion and/or lorazepam may be titrated carefully to reduce consciousness as little as possible while maximising comfort. This should only be considered a treatment of last resort and only after careful discussion with patients/families. [3]
- A single blinded RCT assessed the role of midazolam as adjunct therapy to morphine in the alleviation of severe dyspnoea perception in terminally ill cancer patients. One hundred and one patients with severe dyspnoea were randomized to receive around-the-clock morphine (2.5 mg every 4 hours for opioid-naïve patients or a 25% increment over the daily dose for those receiving baseline opioids) with midazolam rescue doses (5 mg) in case of breakthrough dyspnoea (BD) (Group Mo); around-the-clock midazolam (5 mg every 4 hours) with morphine rescues (2.5 mg) in case of BD (Group Mi); or around-the-clock morphine (2.5 mg every 4 hours for opioid-naïve patients or a 25% increment over the daily dose for those receiving baseline opioids) plus midazolam (5 mg every 4 hours) with morphine rescue doses (2.5 mg) in case of BD (Group MM). All drugs were given subcutaneously in a single-blinded way. In the group with both morphine and midazolam (MM), the proportion of patients with relief from dyspnoea was 92% versus 46% with midazolam alone and 69% with morphine alone. This demonstrates a role for midazolam as an adjunct to morphine in the setting of severe dyspnoea at end of life. [35]
Corticosteroids
- In a recent RCT, high-dose dexamethasone did not improve dyspnoea in patients with cancer more effectively than placebo and was associated with a higher frequency of adverse events. These data suggest that dexamethasone should not be routinely given to unselected patients with cancer for palliation of dyspnoea.[36]
- From clinical experience and biological rationale, the use of steroids for breathlessness due to lymphangitis carcinomatosis or tumour-induced respiratory obstruction may have positive effects, although current evidence is insufficient. [3]
Anti-depressants
- Antidepressants impact on neurotransmitters involved in various brain circuits potentially affecting breathlessness, and are worthy of consideration. Data are mixed for selective serotonin reuptake inhibitors, with positive case series but a negative randomised controlled trial.[3] Mirtazapine is an antagonist at α2-adrenergic, H1, 5HT2A/C and 5HT3 receptors, resulting in serotonin, norepinephrine and dopamine release.
- However the largest multicentre, double-blind randomised, placebo-controlled trial to date found that mirtazapine of doses 15 to 45 mg daily over 56 days did not improve severe breathlessness among patients with COPD or interstitial lung diseases and might cause adverse reactions.[37]
Bibliography
[4] Chronic heart failure in adults: diagnosis and management. NICE 2018. (accessed October 12, 2024).
[7] Muruganandan S, Azzopardi M, Thomas R, Fitzgerald DB, Kuok YJ, Cheah HM, et al. The Pleural Effusion And Symptom Evaluation (PLEASE) study of breathlessness in patients with a symptomatic pleural effusion. European Respiratory Journal 2020;55:1900980.

